Cellulose hydrolysis - cellulases.
Pretreatment opens up the lignocelluose but not to its monomeric form and before it can be used as an energy source it needs to be broken down much more. How does this occur? Enzymes! In particular, one enzyme, cellulase.
Well, actually one class of enzymes; one very large class, grouped together by their ability to hydrolyze the beta 1,4 glycosidic bond (see my last post). One of the reasons for the huge diversity of cellulases is that their substrate, cellulose, comes in many different forms and cellulases have evolved to suit their purpose. If you want to delve into cellulose structure, see this video and this link. Micro-organisms produce many types of cellulases and they work synergistically. The subject is complex and we'll skim the surface.
Cellulases can be classified in a number of different ways depending on what you are interested in: by structure; by sequence; by enzyme mechanism; by substrate etc. One of the most widely used classification systems is the Carbohydrate-active enzymes database, or CAZymes which is based on sequence similarity. In CAZymes, enzymes are grouped into enzymatic groups through their sequence similarity and cellulases fall into the glycosidic hydrolase (GH) group.
 |
| Figure 1. Examples of endo and excoellulase and endogluconasess (link) |
 |
| Figure 2. Cellobiose |
1.Endocellulases bind randomly along a cellulose polymer strand and make several cuts before releasing.
2.Exocellulases (also known as cellobiohydrolases) bind from one end of the cellulose polymer and further bind at either the reducing end or the non reducing end (see my last post on cellulose structure). The polymer strand gets fed into the exocellulose and a D-glucose dimer (cellubiose - figure 2) is cut off, one at a time as the enzyme moves along. This ability - to move along the polymer - is called processivity.
3.Endoglucanases bind to the cellulose polymer, make a cut like an endocellulase and then moves processively along the strand, releasing cellotetraose (figure 3) rather than cellobiose.
4.beta glucosidases cellobiose (and cellotetraose?) into the glucose monomers.
Additionally there are other proteins such as swollenein that insert themselves inbetween the strands of cellulose in crystalline cellulose and help to break it apart.
 |
| Figure 3. Cellotetraose |
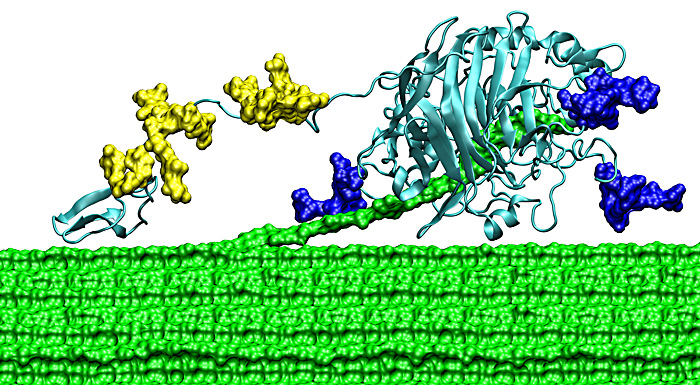 |
| Figure 4. The Family 7 cellobiohydrolase from T. reesei consists of three sub-domains: a small carbohydrate-binding module (CBM); a long, flexible linker decorated with O-linked glycosylation (yellow); and a large catalytic domain (CD) with N-linked glycosylation (blue) and a 50 Å tunnel for the threading of cellodextrin for catalytic cleavage. Cellulose (shown here in green spacefill) is hypothesized to thread into the CD and cleavage occurs at the end of the tunnel. The catalytic product of this enzyme is a disaccharide of β1,4-glucose (cellobiose). (link) |
Two main pathways to cellulose hydrolysis exist: 1. Non-complexed cellusase systems. 2. Complexed cellulase systems (cellulosome).
Lets look at Trichoderma reesei and its non-complexed cellulases. T. reesei is a fungus that has been extensively studied because of its high level of excretion of three cellulases, to the tune of 100g/L. T. reesei has relatively few cellulases (2 exocellulases, 8 endoglucanases and so far 7 beta-glucosidases and I think no endocellulases). Its success as a producer of biotechnology enzymes is due to its high expression under cellulase inducing conditions.The exocellulases are some of the most important enzymes. Cel7a of T. reesei (Figure 4) makes up 60% of the cellulases excreted and degrades cellulose from the reducing end. Cel6a makes up 15-20% and degrades cellulose from the non-reducing end.
The production and purification of cellulases at sufficient levels is one of the main costs of lignocellulose utilization for biofuel and a great deal of research is carried out trying to optimize induction of cellulases, the speed with which they breakdown cellulose and into understanding the regulation of expression. For example, the highest producing cellulase strain of T. reesei in the public domain (i.e. not owned by a company) produces 30g/L of cellulases and is known as RUT30C. It was made by mutagenesis of the parent strain by UV irradiation and subsequent sequencing showed several mutations. One of the most important of these mutations was the truncation of the catabolite repressor protein 1 (cre1). Cre1 represses the expression of cellulases in the presence of more easily metabolizable carbohydrates (catabolites) such as glucose. This is because producing large enzymes such as cellulase is an energetic drain on the micro-organism and only performed when necessary for survival. Therefore turning off the repression of cellulase production, even in the presence of glucose, allows an increased yield of cellulases.
A second area of improvement is in the post translational modifications of the enzyme. If you look at Figure 4, you will notice some yellow blobs attached to the protein. These are sugar molecules that are added during its expression and modified as it is excreted. They help with the expansion of the linker domain that connects the CBM to the catalytic domain (large light blue structure) and increase the reach of the enzyme as it moves along the polymer. Not all micro-organisms "do" glycosylation. For example, bacteria are a favoured hosts for protein expression because they are easier to break open than fungi, grow quickly and can be made to produce high quantities of protein. However, the way in which they glycosylate (add sugars to) proteins is different from that in fungi and even production of cellulase in other model fungi such as S. cerevisae leads to incorrect glycosylation. Lack of correct glycosylation leads to abberrant or less efficient protein production and function.
A third area of improvement is to engineer in cellulases from other organisms. T reesei has a rather lower level beta-glucosidases and these are also inhibited by the end product glucose (2). By engineering the T. reesei to include the beta-glucososidase of another fungus, Aspergillus aculeatus, which is produced at a higher level and which is less susceptible to glucose inhibition, the cellulose hydrolysis rate is much improved.
Thermal stability and rate of cellulose degradation are also aspects that can lead to cost savings in lignocellulose break down.
These are a few of the aspects to consider when contemplating cellulase and their cellulose hydrolysis function. There are also aspects to improve with respect to fermentation and recovery of function cellulases. While T. reesei has been the most studied and utilised organism for biofuel production, much research is also focused on bacterial cellulases and other fungi. In particular, I am interested in the cellulosome....a large and many faceted protein monster...... and what happens to the lignin and hemicellulose? Until next time...
References
1.Beckham GT, J. Ståhlberg, B.C. Knott, M.E. Himmel, M.F. Crowley, M. Sandgren, M. Sørlie, C.M. Payne. Towards a molecular-level theory of carbohydrate processivity in glycoside hydrolases.
Current Opinion in Biotechnology Volume 27, June 2014, Pages 96–106.
2. Tomohisa Hasunuma, Fumiyoshi Okazaki, Naoko Okai, Kiyotaka Y. Hara, Jun Ishii, Akihiko Kondo, A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresource Technology 135 (2013) 513–522.
3. Another good reference: Chapter 6 Hydrolysis of Biomass Mediated by Cellulases for the Production of Sugars