a) Glucose can be depicted linearly in the "Fisher projection" as shown below.
b) In solution glucose adopts a ring structure that can be shown as the Hawthorn projection. When it is in its ring structure it is also known as glucopyranose. Pyranose consists of any structure that has 5 carbons and 1 oxygen in the ring. Glucopyranose is therefore glucose as the 5carbon1oxygen ring structure.
 |
| Glucose in Fisher (top) and Hawthorn projections. Hawthorn projections show α (left) and β (right) conformations (link). |
c)α vs β. You will notice that the glucose Fisher projection can form two types of Hawthorn projections. In the α projection, the OH at C1 points downwards (or is in trans or axial orientation) compared with the CH2OH group whereas in the β projection, the OH at C1 points up (or is in cis or equitorial orientation) compared with the CH2OH group. This is then written as α-D-glucose or α-D-glucopyranose for the trans conformation and β-D-glucose/glucopyranose for the cis conformation. Whether the glucose is α or β affects the binding to the next monomer and the type of enzymes that are able cut the bonds.
d) D vs L. You will also have noticed that I used the letter D.
The letters d and l (lower case) refer to the way in which plane polarised light would be rotated by a chiral center. A chiral center from our perspective is a carbon with for different groups attached. Clockwise rotation means dextroroatorary (d) and anticlockwise rotation is levorotatory (l). Dextro and Levo come from the latin for right and left.
When sugars it gets a bit confusing because D and L (upper case) refers to the actual conformation of the chiral center at the carbon furthest from the carbonyl group in the Fisher projection (i.e. carbon 5) and in which direction the OH group on this carbon is pointing. D means that it is on the right side, L means that it is on the left side. You may also hear them called enantiomers. The D and L enatiomers are mirror images of each other. One particular carbon is referred to because there are often several chiral centers. Glucose has 4 for example. Naturally, only the D form of glucose occurs.
 |
| Fisher projections of L and D glucose from here. * show chiral centers. |
Because of the way in which chiral centers were discovered, (it was based on the conformation of glyceraldehyde), L or D does not always refer accurately to the rotation direction of plane polarised light. For example, amino acids naturally occur in the L-form but for historical reasons it does not follow that this molecule always rotate light anticlockwise. In fact chemists use the R/S system....but we won't go into that.....If you want to dig deeper, google it up and here is the wiki page to get you started. Following any the links from which I have gather images will also give you more information.
e) Joining β-D-glucopyranose together. So, celluose consist of D-anhydroglucopyranose joined together by β-1,4-glycosidic bonds to form an anhydrocellobiose unit. So, lets deconstruct. D means the D form, anhydro, means the loss of the hydroxyl group at the glycosidic bond (I think from carbon4) glucopyranose is the pyranose ring form of glucose.
β means that the OH group at C1 is in the cis conformation and 1,4 means that the glycosidic bond is between carbon 1 of the first glucose unit and carbon 4 of the second glucose unit. Each glucopyranose unit is rotated 180 degrees with respect to the previous one and together they form a repeating unit known has anhydrocellubiose. This is shown below.
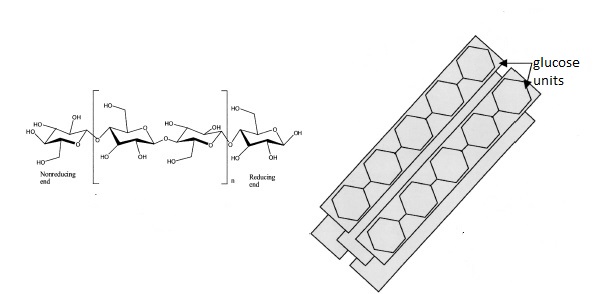 |
| Structure of cellulose featuring anhydrocellobiose unit in the large square brackets (left) and cellulose I crystal structure (right) adapted from ref. 1. |
OK! I think we are ready to tackle cellulases....



No comments:
Post a Comment